Zebrafish: A Preclinical Model for Drug Screening
Preclinical models play a fundamental role in the drug discovery pipeline, enabling researchers to identify compounds of interest, or ‘hits’, through high throughput, drug screening assays. The process screens several compounds against human and/or nonhuman model systems in search of desired biological activity. Ethical and cost concerns lead to use of nonhuman animal models following in vitro hit identification to evaluate toxicology and effectiveness within the complexity of a whole organism. Though imperfect, the use of mice or zebrafish (danio rerio) can help to establish a desirable effectiveness profile at the earliest possible stage.
The Importance of In Vivo Drug Testing
Preclinical drug screening is mainly conducted using in silico, in vitro and in vivo systems. The first two methods can provide vital insights to identify novel compounds eliciting a desired response. Monolayer cultures are excellent at detecting tissue-specific toxicity phenotypes, for instance. New 3D cell culture methods also permit study of how neighbouring cells can affect the response to pharmaceuticals. However, they cannot provide the level of depth necessary to understand more complex mechanisms arising from interactions between multiple tissues. For that, in vivo animal models are required.
Mice are the eponymous animal model used for clinical trials and biomedical research. Among the many reasons for their unique suitability to laboratory settings is their physiological similarity to human beings. Yet the zebrafish embryo has disrupted the hegemony of using mammalian animal models for studying human disease etiology and carrying out drug screening.
Why Use Zebrafish Models Instead of Mice?
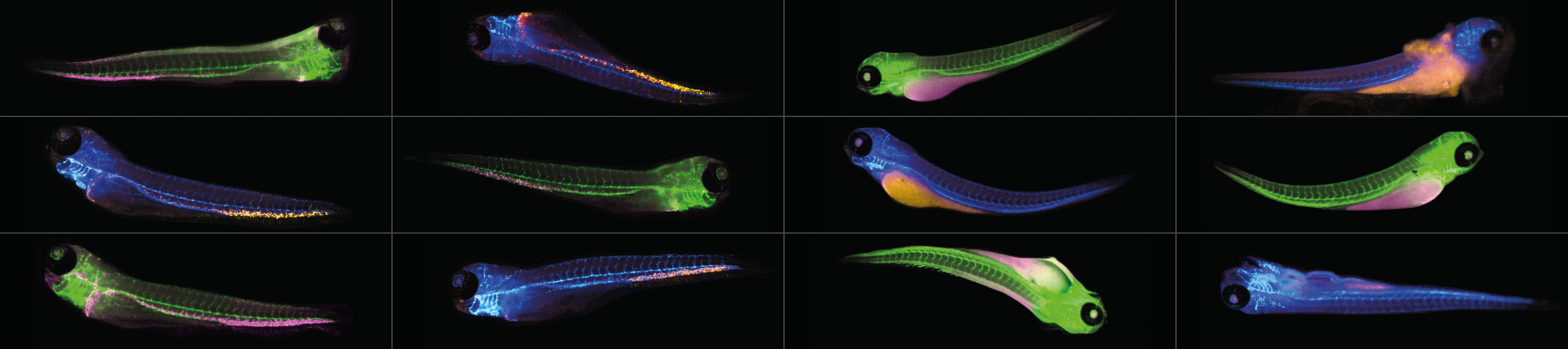
Chemical screening in zebrafish is a powerful method of studying small molecule interactions across living multiorgan systems. Observables include compound toxicities, metabolic alterations, pharmacokinetics, and cell niche modulations. Although mice have a closer evolutionary similarity to humans, comparisons have shown that approximately 70% of human genes have at least one zebrafish orthologue [3]. Additionally, zebrafish offer a wide range of beneficial characteristics which make them an exceptional platform for cancer and therapeutic research. They are smaller than mice and hundreds can be housed in shoals, leading to immediate space-and-cost savings. The zebrafish genome is amenable to CRISPR-based genetic modification for introduction of cell-specific read-outs, such as fluorescence. Plus, their reproductive cycle is on the scale of days rather than weeks and may produce hundreds of eggs at a time. These advantages lead to ever growing libraries of specialized zebrafish lines that are easier and cheaper to maintain than mice.
However, the benefits of zebrafish screening go beyond mere operational advantages. Zebrafish embryos are an extraordinary platform for drug testing owing to their optical transparency. Fluorescently labelled tissues can be directly visualized in transgenic zebrafish embryos using a range of imaging modalities. And many organs can directly be visualized using transmitted light for label-free imaging studies. The benefits for microscopy are amplified by the size of the embryos, being able to fit within multi-well plates to potentially increase experimental throughput.
Challenges of Zebrafish Screening
While zebrafish offer many benefits for drug screening assays, there are significant challenges when it comes to high throughput screening. When using microscopy as a tool, it can be difficult to automate the data acquisition and analysis workflows. To be useful, the zebrafish embryo must be properly positioned to obtain informative images. Otherwise, the anatomy of interest may not entirely be visible or within the focal plane visualized. Currently, it is common for researchers to manually place embryos into desired lateral or dorsoventral orientations one at a time prior to imaging.
When imaging large numbers of zebrafish, a major bottleneck arises when looking to automate image analysis. Use of multi-well plates compounds the scale of the challenge. The first difficulty is that these fish are living, three-dimensional objects. So, even when anesthetized, they can roll and drift about, making it unlikely they will maintain a perfect positioning within the wells. In a large-scale automated screen, it is impractical to manually go through the images to exclude those fish that are improperly positioned.
Another critical image analysis challenge is the ability to examine one specific anatomical region of the zebrafish embryo, while ignoring others. For example, when looking at cells in the tail, standard fluorescence analysis using thresholding picks up bright spots elsewhere in the fish, either from cells located outside the anatomy of interest or from autofluorescence. Currently, it is commonplace to either ignore the visible anatomy and simply analyse fluorescence images through intensity thresholding, or to manually select the region of interest. The former reduces the information extracted from each embryo while the latter makes automated workflows impossible and reduces the size of drug libraries that can be studied.
For ideas about how new technologies are overcoming these challenges, and how they can be assembled into automated workflows, check out our recent perspectives article in SLAS Technology.
Solutions for Zebrafish Screening
At IDEA Bio-Medical, we offer a novel deep learning-based image analysis solution for in vivo zebrafish screening. It is an affordable, user-friendly system designed specifically for rapid zebrafish image-based screening. If you would like to learn more about the system, read our application note on automated, deep-learning based analysis of zebrafish. Or contact a member of the team directly.
